Experimental trials with GMOs (other than plants): Regulatory procedures
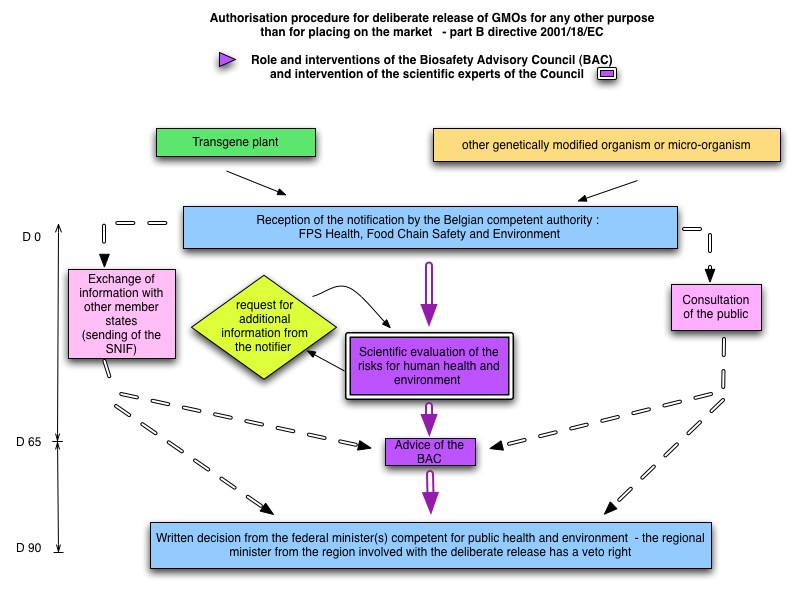
The roles and interventions of the Council in the regulatory procedure for notifications submitted for the deliberate release of a GMO for any other purpose than for placing on the market under Part B of the Royal Decree of 21/02/2005 implementing Directive 2001/18/EC are summarised in the diagram on the right side.
For the scientific evaluation of these dossiers and in addition to the abovementioned Decree, the Council can refer to several guidelines applicable to GM (micro-)organisms, listed on the Belgian Biosafety Server.